How to safely monitor your small animal neurology patients during MRI can feel overwhelming, especially when faced with tricky cases. Elizabeth Goudie DVM, MS, DACVAA, discusses how – with some simple planning and the right monitoring – we can help alleviate some of these anxieties when presented with an MRI patient, and work to improve patient care.
Why careful monitoring matters during MRI
Small animal neurology patients undergoing MRI often present with conditions such as intervertebral disc disease, cervical spine disorders, or intracranial lesions. Even those with seemingly minor neurological issues may have comorbidities that increase anaesthetic risk. Understanding how to safely monitor these patients during MRI is key to optimal outcomes.
MRI procedures limit hands-on monitoring, making anaesthesia management challenging and sometimes overwhelming for even the most seasoned of anaesthetists. With strategic planning and MRI-compatible monitoring equipment, veterinary teams can reduce risk and improve patient outcomes.
Successful preparation is essential to safely monitoring your patient during an MRI and should include:
- Providing heat on the induction table
- Obtaining a baseline ECG and blood pressure
- Preoxygenate all patients for 3-5 minutes before intubation.
- Using a multiparameter monitor during the scan
- Maintaining normothermia at induction and during preparation will help prevent hypothermia in the MRI
- Closely monitoring the patient in the recovery period
Perhaps, most importantly, all abnormalities and concerns should be communicated between the dedicated team members. This includes a dedicated anaesthetist, a dedicated imaging technician, and the case doctor. Rounds before the case and discussion of any patient concerns during the imaging process help alleviate any miscommunications that could negatively impact the patient’s outcome.

Pre-anaesthetic assessment for MRI patients
When preparing for an anaesthetic episode, treat the MRI patient as you would any patient undergoing surgery or any other procedure. A thorough review of the patient’s history and recent bloodwork is essential. A good rule of thumb is the availability of blood test results within the month (if no significant medical changes have occurred recently) and diagnostics. A perianaesthetic checklist should also be incorporated.
- Physical examination: evaluate the patient’s cardiovascular, respiratory, and neurological systems to identify any underlying conditions that could affect anaesthesia, including any history of metallic implant/foreign body
- Diagnostic tests: perform pre-anaesthetic blood work (e.g., CBC, biochemistry panel) to assess organ function and identify any abnormalities
- Risk stratification: classify the patient’s anaesthetic risk (e.g., ASA status) to tailor the anesthesia protocol
Multiparameter monitoring
It is important to monitor our small animal neurology patients at the same level as we monitor our other patients, particularly those patients with intracranial lesions. Anaesthesia quickly disrupts homeostasis, and having a way to closely watch for disruptions, especially in higher ASA status patients, is vital because we are unable to place our hands on our patients during the scan. Minimum recorded parameters should be:
- Every 5 minutes: Heart rate, respiratory rate, end-tidal carbon dioxide, and blood pressure
- Every 15 minutes: Pulse oximetry and temperature
While recording occurs every 5- 15 minutes, all monitoring should be applied to the patient and observable throughout the procedure.
ECG monitoring
While the use of this modality is prone to artefacts, its use is still recommended:
- Obtain a baseline ECG before inducing to ensure no underlying arrhythmias
- Artifacts are due to magnetohydrodynamic (MHD) effects that alter the ECG signal, as well as magnetic field gradient switching
- Between scans or during less disruptive scan sequences, it is a reliable modality
Bradyarrhythmias are not uncommon in small animal neurology patients undergoing MRI. They can occur due to several factors, including increased intracranial pressure, which affects autonomic regulation; breed predispositions such as in Dachshunds; the use of certain drugs like opioids and dexmedetomidine that depress heart rate; and hypothermia, which commonly develops during prolonged anaesthesia and imaging procedures.
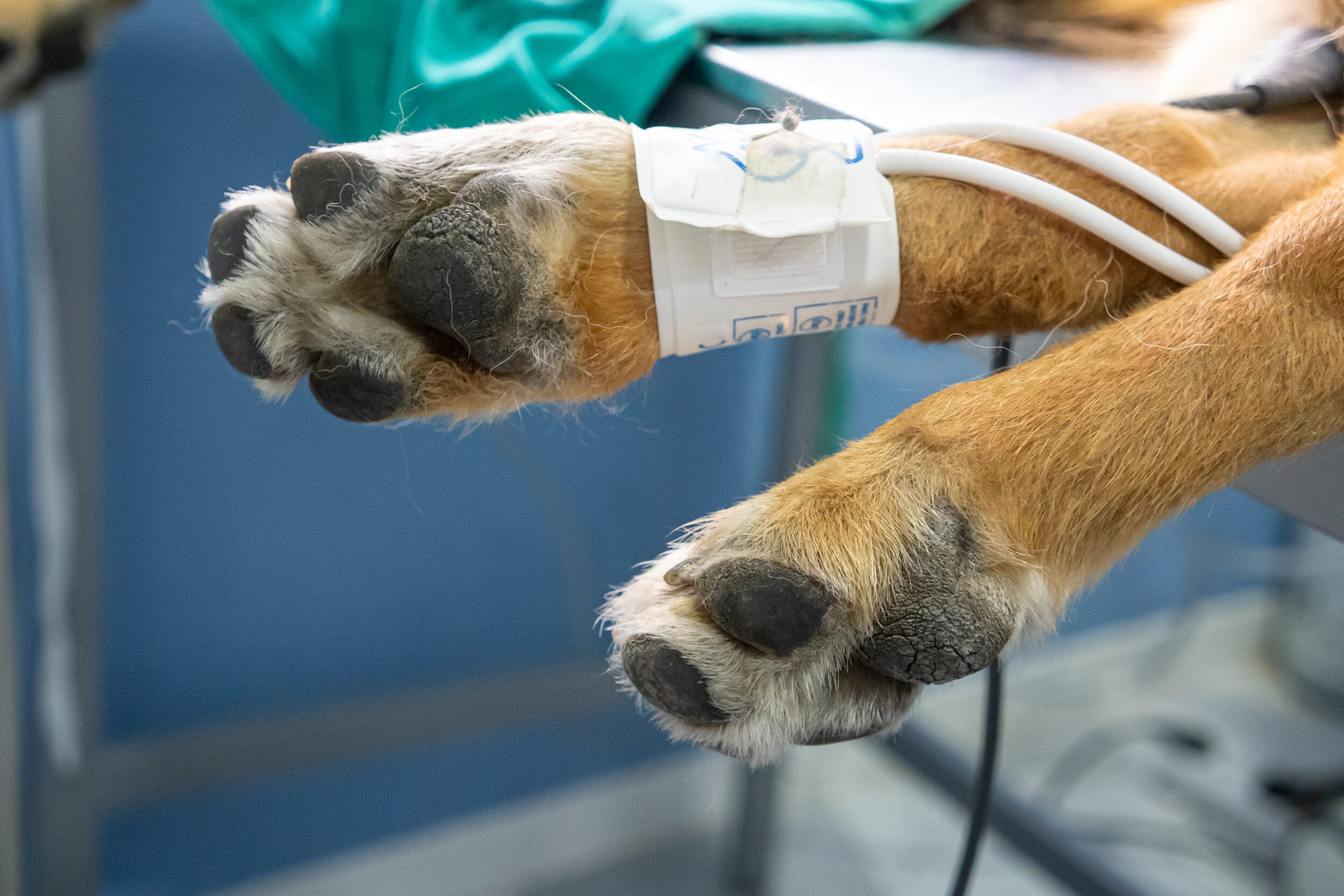
The use of MRI-specific leads, including MRI-specific adhesive ECG pads, should be adopted to prevent injury, damage to the MRI, or artefact.
- Cables and fluid lines should never be looped
- Cables should not be against non-haired skin
- Cables need to be run parallel to the bore of the magnet
Monitoring pulse oximetry (SpO2)
Pulse oximetry offers a way to monitor heart rate when an ECG or invasive blood pressure heart rate is unavailable. Artefacts do occur, but less consistently than with ECG.
- The plethysmograph (pulse oximetry waveform) can also provide valuable information. Pulse pressure variation can show the need for fluid resuscitation.
- Changes to the waveform can indicate arrhythmias that may not be observable on ECG due to artifact
- Covering the probe insulates it from ambient light and can improve results
- When placing the pulse oximeter, consider other locations besides the tongue.
- For probes that wrap, shaving the ventrum of the tail and placing the probe around the tail prevents the tourniquet effect – or slipping – that is often observed when using the tongue.
- Ensure the probe used is MRI-safe to avoid artefacts

Monitoring blood pressure
When monitoring blood pressure in small animal neurology patients, some monitors are more reliable than others when it comes to oscillometric measurements:
- Invasive blood pressure availability: gaining proficiency in arterial line placement is recommended, and arterial line invasive blood pressure monitoring for small and/or critically ill patients should be considered
- Many facilities will place arterial lines for all MRI scans to ensure accurate readings
Blood pressure is especially important to monitor when it comes to the Cushing Reflex. This is a physiological response to increased intracranial pressure (ICP) characterised by the Cushing triad: hypertension, bradycardia, and irregular respirations, often indicating impending brain herniation. Remember, under anaesthesia, abnormal respirations may not be noted.
Monitoring end-tidal CO2
This modality is probably our most reliable when it comes to artifacts during the MRI scan.
- It is vitally important to monitor when there is a risk of ICP elevations: End-tidal CO2 should be maintained at 35-40 mmHg for patients with increased intracranial pressure
- For all other patients, it is satisfactory to target normocapnia (35-45 mmHg) or mild permissive hypercapnia (<55 mmHg)
- Many patients anaesthetised for MRI are mechanically ventilated; capnography is used to help tailor the ventilator settings
- If the patient will spontaneously ventilate, and the MRI scan is not negatively impacted, I prefer to allow the patient to do so in order to maintain blood pressure/venous return and ensure continued spontaneous ventilation at the end of the MRI. This action will help speed the recovery process.
MRI-compatible equipment
When it comes to safely monitoring your small animal neurology patients during an MRI scan, it’s a good idea to research compatible equipment such as ventilators, monitors, fluid pumps, and heating. This is by no means an exhaustive list, but some of those to consider are:
Ventilators
There are many MRI-safe ventilators available, including (but not limited to):
- Hamilton Medical: a newer, MRI-compatible ventilator allowing for easier settings with multiple advanced ventilation mode settings
- Penlon-Nuffield: a pneumatically driven ventilator which may – initially – be harder to learn to use
- Hallowell: a simple volume cycled ventilator that many veterinary staff are comfortable with
- Smiths: a pneumatically driven volume cycled ventilator that has an MRI conditional model
Monitors
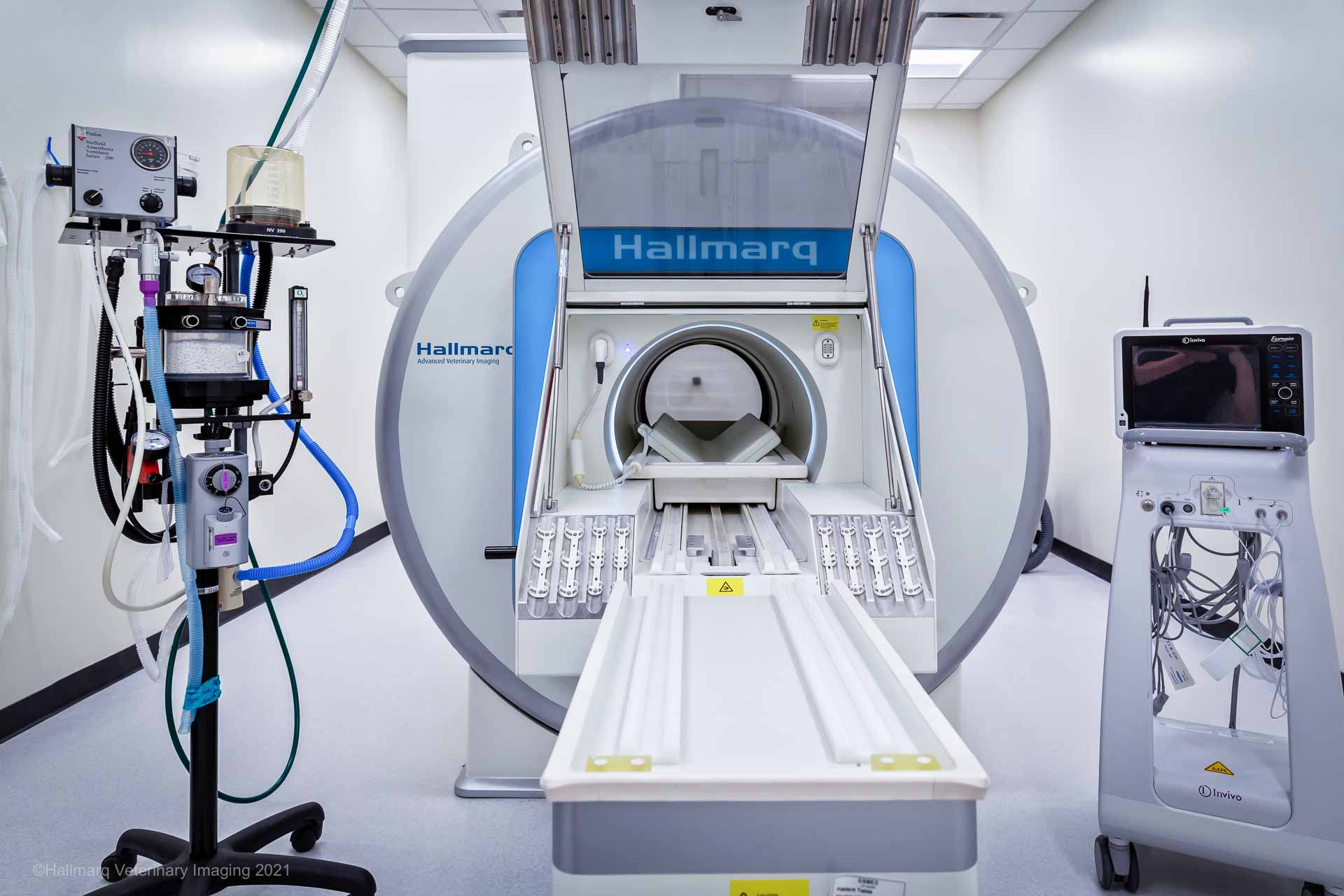
For ‘traditional’ small animal MRI, a monitoring system outside of the MRI room can be added using a shielded waveguide. However, for veterinary-specific, small animal 1.5T MRI, such as MIRA from Hallmarq, a shielded waveguide is unnecessary. The system incorporates a unique built-in RF shield enabling an MRI-safe setup in an already established space. However, an MRI-safe monitor or an MRI-safe set-up should be assessed and put into place, whichever option is chosen.
MRI-safe monitors include:
- Iradimed: A reliable monitor that remains in the room with the patient. A separate tablet is placed in the anteroom. Multiparameter, including the ability to add invasive blood pressure
- Digivet (Digicare) LifeWindow. A veterinary-specific monitor with MRI-safe/compatible connections, with the monitor located outside the MRI room. Cords are passed through the waveguide
- SAII Model 1035 MR compatible: A veterinary-specific monitor providing a combination of in-room and outside, with the monitoring module outside the MRI room and parameters on a PC screen. ECG does NOT require shaving for the pad
- Tesla M3: An MRI-safe monitor that remains in the MRI room with a remote MRI monitor. A multiparameter includes IBP and anaesthetic gas monitoring
- Phillips: The Invivo is the older model of the company’s MRI monitors. Non-invasive blood pressure can be unreliable due to small patient size and often requires invasive blood pressure in very small, vasoconstricted patients. Their new anaesthesia monitor, Expression, is also worth considering.
Fluid pumps
Fluids should be provided for these MRI patients. Partial intravenous anaesthesia or total intravenous anaesthesia can be administered either within the patient’s IV fluid bag or in a smaller bag, both using standard administration sets. Alternatively, MRI-safe syringe pumps or Faraday cages can be used to allow for small-volume CRIs.
While there are MRI-compatible IV fluid pumps (Iradimed and MedRad) many hospitals will have a standard pump outside the MRI room with extensions through the wall or outside of the 5-gauss line.
Heating
Many of the monitors listed here allow for surface or corporeal temperature monitoring. The maintenance of normal body temperature in MRI greatly improves the anaesthetic episode. It maintains a normal heart rate and minimises peripheral vasoconstriction, which allows for more accurate NIBP measurements. Aside from optimising MRI anaesthesia, a normothermic patient will have a better surgical outcome, including minimising coagulopathies and infection.
Finally, a normothermic patient will recover more quickly and smoothly than a hypothermic one. Luckily, Hallmarq’s small animal 1.5T MRI includes a self-shielded RF hatch, so it has the benefit of maintaining temperature more reliably than other systems. Recommendations for maintaining temperature include a heated table at induction during prep for MRI, blankets for maintaining temperature in the MRI, and the use of ConRad thermal blankets, which have been remarkably reliable in maintaining temperature.
How to deal with an anaesthetic emergency during MRI
If you are concerned that your patient is decompensating in the MRI, the patient should be immediately moved out of the magnet and into the anteroom. A crash cart (a tacklebox is usually adequate) should always be available as standard:
- This tacklebox should not be used outside of emergencies and should be checked for expiration regularly and instrumented with a lock to ensure it is well-stocked at all times
- The location of the defibrillator should be known and should be accessible to bring to the MRI area
- Extra endotracheal tubes, an MRI-compatible laryngoscope, reversal drugs, and CPR drugs should be included (see Fig 1. below for drugs available)
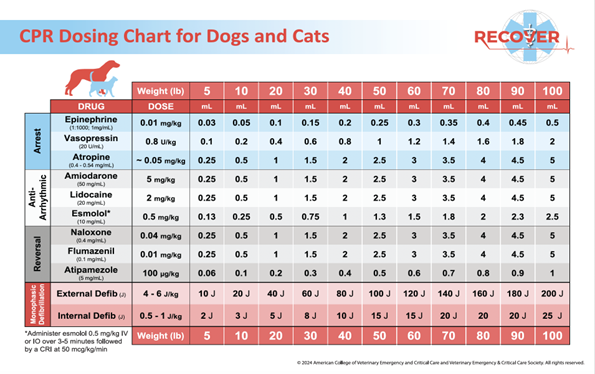
Source: American College of Veterinary Emergency and Critical Care and Veterinary Emergency and Critical Care Society (© 2024).
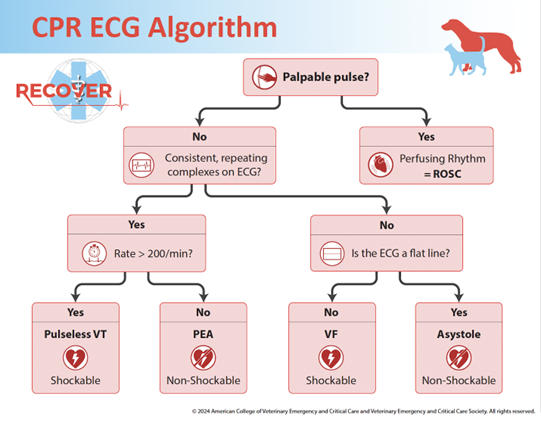
Source: American College of Veterinary Emergency and Critical Care and Veterinary Emergency and Critical Care Society (© 2024).
Post-anaesthetic complications
All patients should have TPRs monitored until extubated, normothermic, and QAR, and allowed to recover in sternal recumbency to improve oxygenation and prevent aspiration. Complications may include:
- Hypothermia: will cause delayed recovery, and patients may require drug reversal. Ensure active warming is provided
- Pain: a full mu: partial Mu agonist, NSAID, or NMDA antagonist can be provided. Consider local blocks if possible, during surgery
- Dysphoric recovery: consider pain as a differential. Consider reversing opioids if the surgery is non-painful or planned. Butorphanol or Naloxone are options for pure mu opioids; Naloxone will reverse Butorphanol. Sedation may be needed: Acepromazine 0.005-0.01 mg/kg IV or Dexmedetomidine 0.5-1 ug/kg IV
- Delayed recovery: check rectal temperature to ensure the patient is not hypothermic. If ICP is increased, consider hypertonic saline or mannitol. Consider reversal if non-painful or no surgery planned
Key takeaways for small animal neurology specialists
With proper preparation, MRI anaesthesia can be safe, efficient, and stress-free. Using MRI-compatible monitoring systems, maintaining normothermia, and clear team communication ensure better outcomes for small animal neurology patients.