Elizabeth Goudie DVM, MS, DACVAA, discusses factors to consider when anaesthetising the small-animal MRI patient. This post focuses solely on ASA II patients; those considered otherwise healthy, requiring an MRI for musculoskeletal disease or intervertebral disc disease and with no other major comorbidities
How should I prepare a small animal patient for MRI anaesthesia?
Firstly, when preparing for an anaesthetic episode, treat the MRI patient as you would any patient undergoing surgery or other procedure. A thorough patient history and recent bloodwork (within the month if no significant medical changes have occurred recently), and diagnostics should be obtained. A thorough medical history can be used to evaluate the risk of metallic foreign material, and a survey radiograph performed to confirm this. Aside from the risk to the patient, a metallic object can alter the diagnostic quality of the MRI.
Successful preparation includes providing heat on the induction table and obtaining a baseline ECG and blood pressure. Additionally, preoxygenate all patients for 3-5 minutes before intubation. Maintaining normothermia at induction and during preparation will help prevent hypothermia in the MRI.
What anaesthetic monitoring should I have for the small animal MRI patient?
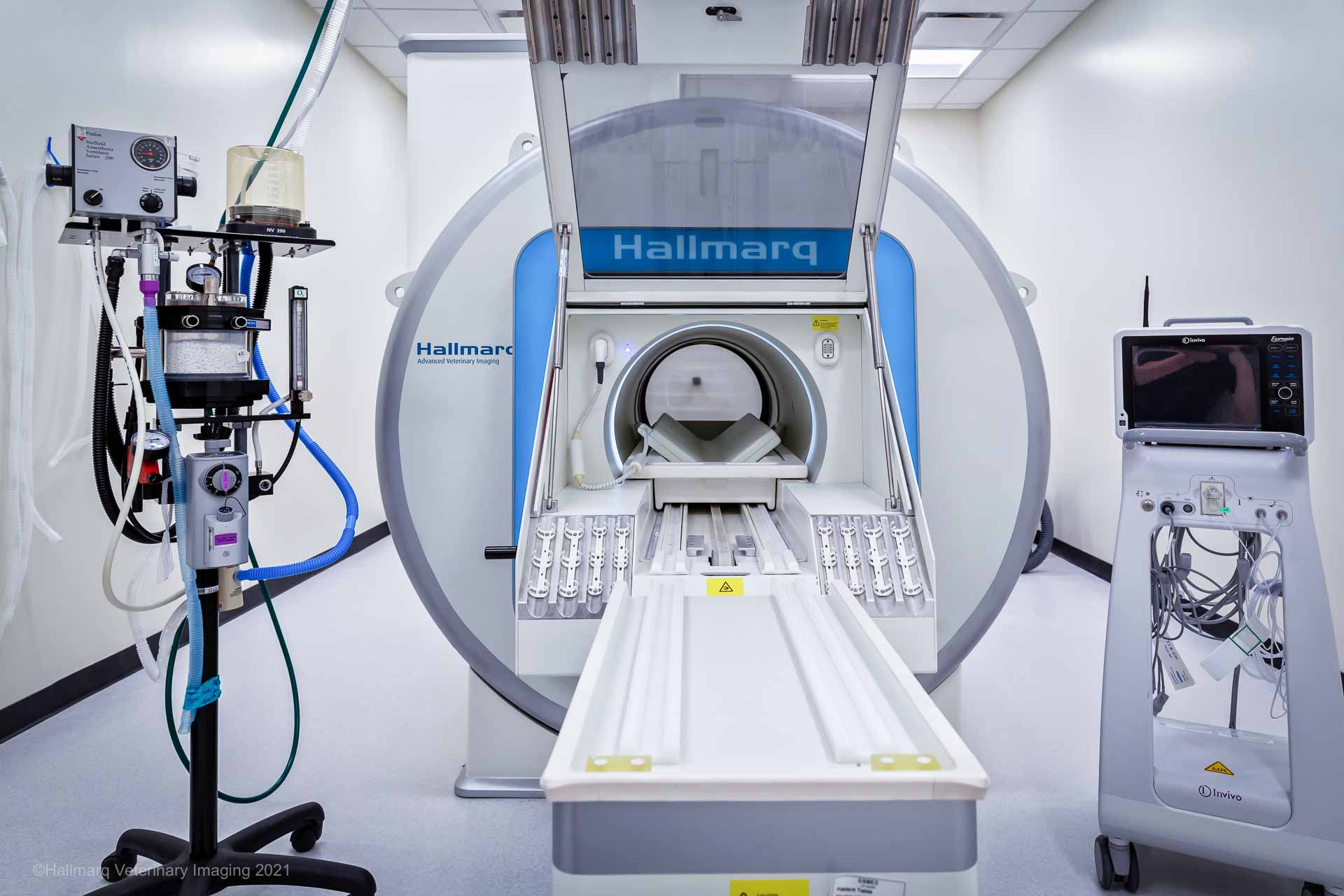
It is vital to have full monitoring placed for all patients just as they would have during a surgical procedure. This should include: a multiparameter monitor with ECG, non-invasive blood pressure (NIBP), pulse oximetry, and capnograph. A functioning multiparameter monitor is even more important in the MRI with the limited access typically allowed to the patient throughout the scan. Checking your patient during the scan is easier with the Hallmarq Small Animal 1.5T MRI. Their unique self-shielded hatch allows free access to the MRI room and incorporates video monitoring for increased patient safety.
When choosing a monitor, there are a couple of options. There are monitors that are MRI-compatible with a unit in the room, or multiparameter monitors that are in the anteroom with MRI-safe wires that snake through waveguides. Invasive blood pressure monitoring is well worth the investment, especially for small and critical patients.
What are some common anaesthetic abnormalities in small animal MRI?
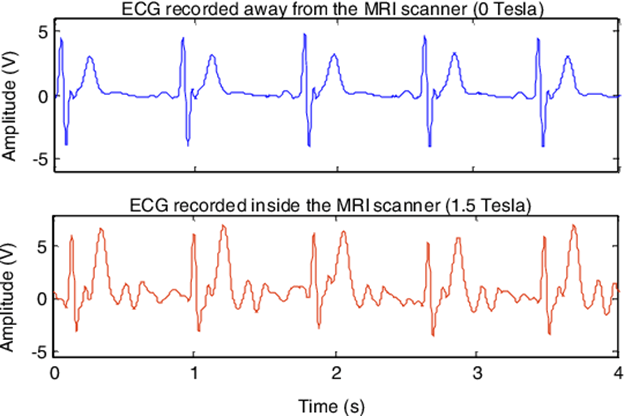
ECG abnormalities are common during the MRI, which is why it is important to obtain a baseline ECG. While arrhythmias can occur with a normal baseline ECG, it can help to differentiate underlying pathophysiology from MRI-generated abnormalities. ECG artefacts are due to magnetohydrodynamic (MHD) effects that alter the ECG signal, as well as magnetic field gradient switching. The MRI sequence can be paused to check for a return to a normal rhythm, but the MHD effects can remain. These will affect the measured heart rate and appearance of the S-T segment.
Another commonly encountered complication is NIBP measurement. A baseline blood pressure and appropriate measurement of cuff size and placement (proximal to the tarsus or carpus, or at the base of the tail) will help ensure appropriate readings. Changing the setting on the monitor to pediatric or neonate for most patients helps ensure appropriate readings.
Avoiding hypotension and dehydration
While it is easy to ignore abnormalities on the monitor, there are some abnormalities I have found more likely to be real. Many of my patients do become hypotensive (this is based on invasive blood pressure monitoring). I believe this is primarily due to hypothermia and lack of sympathetic stimulation. Having the patient at the lowest inhalant setting, and maintaining a normal heart rate, is often adequate to ensure maintenance of a MAP of at least 60 mmHg.
Many otherwise healthy IVDD patients with intervertebral disc disease are dehydrated (loss from panting, lack of intake, etc.). They will benefit from a 5-10 mL/kg isotonic crystalloid bolus before induction of anaesthesia. Further, the addition of a MAC-sparing CRI (fentanyl or butorphanol) in the fluid bag can help minimise inhalant requirements to maintain normal vascular tone. Discussion on how to create MAC-sparing CRIs or the addition of vasoactive CRIs is outside the scope of this article.
Preventing hypoventilation in the small animal MRI patient
Unless a patient requires ventilation for the prevention of intracranial pressure risk, I generally allow my patients to ventilate on their own. An end-tidal carbon dioxide of 50 mmHg is acceptable assuming an otherwise healthy patient with no underlying acidemia or elevated intracranial pressure (ICP). This helps maintain my blood pressure by preventing compression of the caudal vena cava. It also ensures I maintain my patient’s depth to prevent hypoventilation. If a patient requires mechanical ventilation, I carefully assess my plethysmograph waveform for pulse pressure variation. I then administer a fluid bolus if it is observed to maintain intravascular volume.
When placing the pulse oximeter, consider other locations besides the tongue. For probes that wrap, consider shaving the ventrum of the tail, and placing the probe around the tail. This prevents the tourniquet effect or slipping, often observed when using the tongue.
Minimising heat loss in the small animal MRI patient
Finally, thermoregulation is a major concern during MRI. Induction on a table with heat (forced air or circulating heat blanket) can help ensure a normothermic patient when entering the MRI. The majority of heat loss occurs within the first 45 minutes of anaesthetic induction.
Hallmarq’s Small Animal 1.5T MRI prevents some heat loss by maintaining the patient within a closed unit that prevents some convective heat loss. Evaporative, radiative, and conductive loss is still present. The addition of a thermal barrier blanket/pad to the patient can assist with radiative and convective heat loss. Placing a barrier between the patient and the table can minimise conductive heat loss.
What steps should I take in an emergency with small animal MRI?
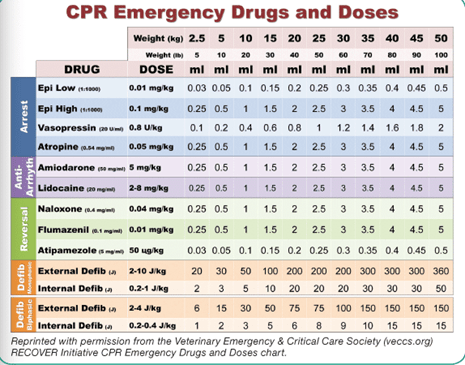
If you are concerned that your patient is decompensating in the MRI, the patient should be immediately moved from the magnet and into the anteroom. A crash cart (a tacklebox is usually adequate) should be available. This tackle box should not be used outside of emergencies and should be checked for expiration regularly. Furthermore, it should be instrumented with a lock to ensure it is well stocked at all times. Extra endotracheal tubes, an MRI-compatible laryngoscope, reversal drugs, and CPR drugs should be included.